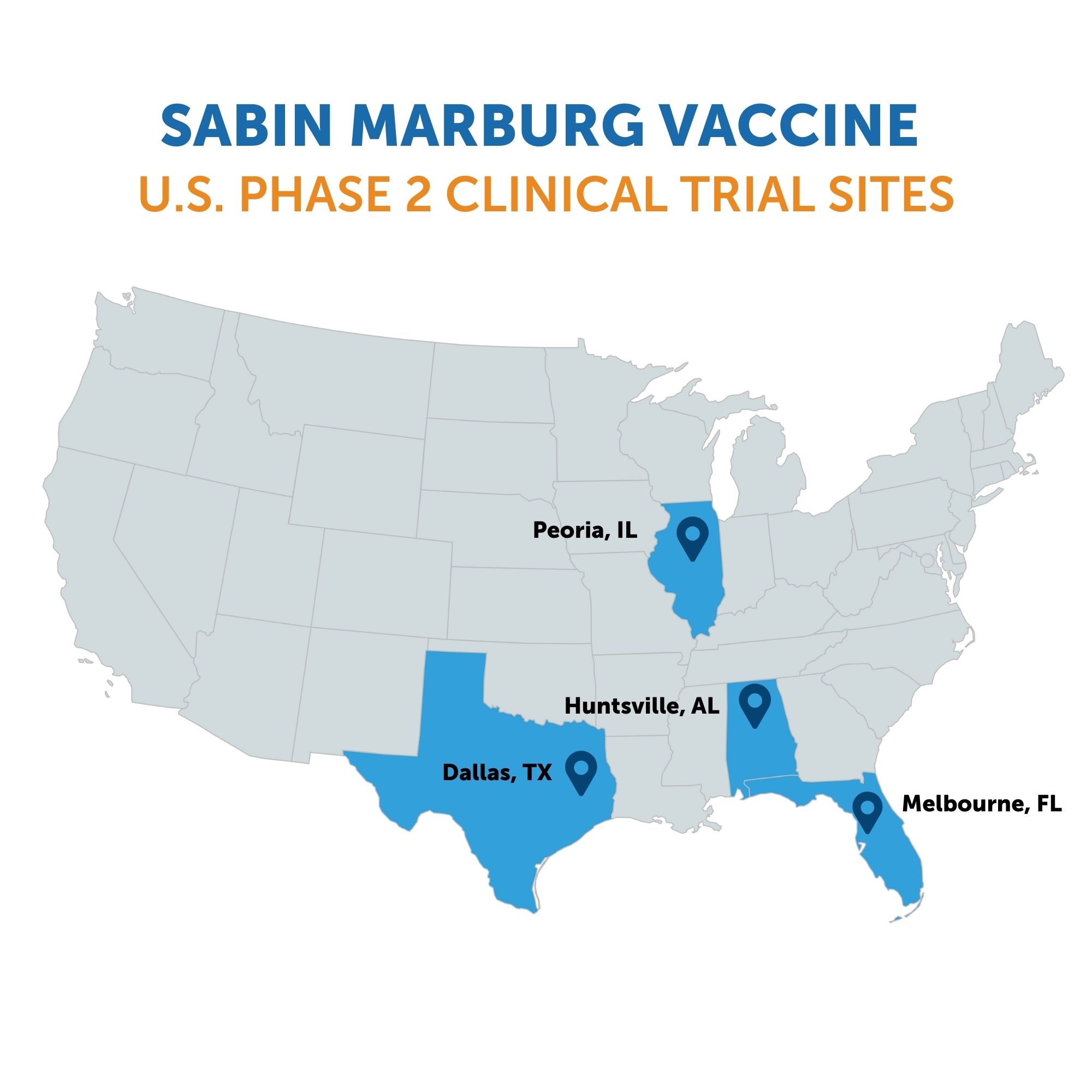
WASHINGTON, April 16, 2025 (GLOBE NEWSWIRE) -- The Sabin Vaccine Institute has launched a multi-site Phase 2 clinical trial in the U.S. for its Marburg vaccine candidate, administering doses to the first participants in Melbourne, Florida. This trial builds on ongoing Phase 2 testing in Kenya and Uganda, with initial findings from that research expected in the coming months.
Sabin's vaccine development efforts, including clinical trials, are becoming increasingly critical as Marburg outbreaks grow in frequency, underscoring the urgent need for vaccines to protect those at highest risk. Sabin supported an open-label Phase 2 clinical trial sponsored by the Rwanda Biomedical Centre (RBC) by supplying the investigational vaccine during Rwanda's 2024 Marburg outbreak. More than 1,700 individuals — primarily frontline health care workers — were vaccinated, with first doses arriving within nine days of the outbreak being declared. Data from the RBC trial will be shared with Sabin to support the vaccine's licensure.
Rwanda's outbreak ended on December 20 with a case fatality rate of 23%, lower than the historical average of 50%. Fatality rates in outbreaks can vary due to multiple factors, including greater surveillance, prompt detection, supportive care, and the overall response effort. On January 20, Tanzania declared an outbreak of Marburg virus disease, which ended on March 13.
Currently, there are no approved vaccines for Marburg virus disease.
For the U.S. clinical trial that began this week, Sabin will recruit 200 volunteers aged 18 to 70 across four locations – in addition to Melbourne, the vaccine will be tested at sites in Dallas, Texas; Huntsville, Alabama; and Peoria, Illinois. The randomized, placebo-controlled, double-blind trial will continue to evaluate safety and immunogenicity, and will monitor vaccinated volunteers for a year.
“Recent outbreaks highlight the urgent need to strengthen our defenses against this deadly and unforgiving disease,” says Amy Finan, Sabin's Chief Executive Officer. “Sabin's Phase 2 clinical trials will generate essential data to move this vaccine closer to licensure — and offer a potentially life-saving tool where none exists.”
Marburg is a filovirus, in the same family as the virus that causes Ebola. Like Ebola, Marburg virus disease spreads via direct contact with the blood or other bodily fluids of infected individuals. It is highly virulent and causes hemorrhagic fever.
“Conducting clinical trials in Africa is key to evaluating the vaccine in regions where Marburg and other filoviruses are most common or endemic," notes Kelly Warfield, Sabin's President of Research & Development. "The U.S. trial will give us vital safety and immune response data for non-endemic populations, helping us better prepare for outbreaks and spread of this disease.”
Based on the cAd3 platform, Sabin's single-dose investigational Marburg vaccine was found to be promising in Phase 1 clinical and non-clinical studies, with results showing it to be safe, while eliciting rapid and robust immune responses.
The Marburg vaccine trials are supported by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, under multi-year contracts between the organizations.
BARDA and Sabin began working together in September 2019 to develop monovalent vaccine candidates for Marburg virus and Sudan virus diseases. To date, Sabin has received around $252 million in contract awards from BARDA for furthering vaccine research and development against these two disease threats.
A Phase 2 clinical trial for Sabin's Sudan virus vaccine is underway in Uganda and Kenya. The cAd3 Sudan vaccine candidate will also be tested among adult volunteers in the U.S. later this year.
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Center for the Biomedical Advanced Research and Development Authority (BARDA), under contract numbers 75A50119C00055 and 75A50123C00010.
For information about Sabin's Phase 2 Marburg vaccine trials, visit:
Clinicaltrials.gov -- NCT06620003 (U.S. trial)
Clinical.trials.gov -- NCT05817422 (Uganda and Kenya trial)
Pan African Clinical Trials Registry -- PACTR202306534727467 (Uganda and Kenya trial)
About the Sabin Vaccine Institute
The Sabin Vaccine Institute is a leading advocate for expanding vaccine access and uptake globally, advancing vaccine research and development, and amplifying vaccine knowledge and innovation. Unlocking the potential of vaccines through partnership, Sabin has built a robust ecosystem of funders, innovators, implementers, practitioners, policy makers and public stakeholders to advance its vision of a future free from preventable diseases. As a non-profit with three decades of experience, Sabin is committed to finding solutions that last and extending the full benefits of vaccines to all people, regardless of who they are or where they live. At Sabin, we believe in the power of vaccines to change the world. For more information, visit www.sabin.org and follow us on X @SabinVaccine.
About Sabin's Vaccine R&D and the cAd3 Platform
In August 2019, Sabin announced exclusive agreements with GSK for Sabin to advance the development of the prophylactic candidate vaccines against the deadly Zaire ebolavirus, Sudan virus, and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, which was acquired by GSK in 2013. The candidate vaccines, based on GSK's proprietary cAd3 (Chimpanzee Adenovirus Type 3) platform, were further developed by GSK, including the Phase 2 development for the Zaire ebolavirus vaccine. Under the agreements between GSK and Sabin, Sabin exclusively licensed the technology for all three candidate vaccines and acquired certain patent rights specific to these vaccines.
Media Contact:
Monika Guttman
Senior Media Relations Specialist
Sabin Vaccine Institute
+1 (202) 621-1691
press@sabin.org
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cdb8a6cd-2ff2-49bd-aeb4-43e859c1b44f
-
Sabin Begins Marburg Vaccine Trial in U.S.WASHINGTON, April 16, 2025 (GLOBE NEWSWIRE) -- The Sabin Vaccine Institute has launched a multi-site Phase 2 clinical trial in the U.S. for its Marb2025-04-17
-
Treasure Global Inc. Secures Exclusive Rights to Launch Mezzofy’s USD40 Billion Coupon Platform in MKUALA LUMPUR, Malaysia, April 16, 2025 (GLOBE NEWSWIRE) -- Treasure Global Inc. (NASDAQ: TGL) (“Treasure Global” or the “Company”), is pleased to2025-04-17
-
20 万境外采购商云集广交会,警惕 “山寨邮箱”“冒充老板” 等诈骗套路第137届广交会于4月15日拉开帷幕,来自215个国家和地区的超20万名境外采购商预注册。此外,255家全球零售250强和各国各地区大商将组团参会,创历史新高。这场国际贸易2025-04-17
-
电影《寻找英雄》全国上映 李梦男再现精湛演技由王军导演,李梦男主演的电影《寻找英雄》即将全国上映,2025年4月16日电影《寻找英雄》在北京华夏电影中心举办电影首发仪式。 电影首发现场,国家革命老区建2025-04-17
-
刘诗诗《掌心》上星达成上星率100%,勇于突破客串张艺谋新作《惊蛰无声》近日,刘诗诗再次成为焦点,不仅因为最近的Celine活动刷屏,更有主演的电视剧《掌心》上星浙江卫视黄金档,同时官宣特别出演张艺谋导演的新片《惊蛰无声》,让我们一同走进2025-04-17
