Magstim Passes Compliance with Strict European Union Medical Device Regulations
Expanding Magstim Access to Entire EU Marketplace
WHITLAND, United Kingdom, May 16, 2025 (GLOBE NEWSWIRE) -- Magstim has been granted EU MDR Certification for all transcranial magnetic stimulation (TMS) systems, demonstrating compliance with strict European Union Medical Device Regulations. This provides a path for Magstim TMS technology to be sold throughout the EU marketplace, and proves that the device is safe, clinically effective and has passed all requirements for testing and traceability.
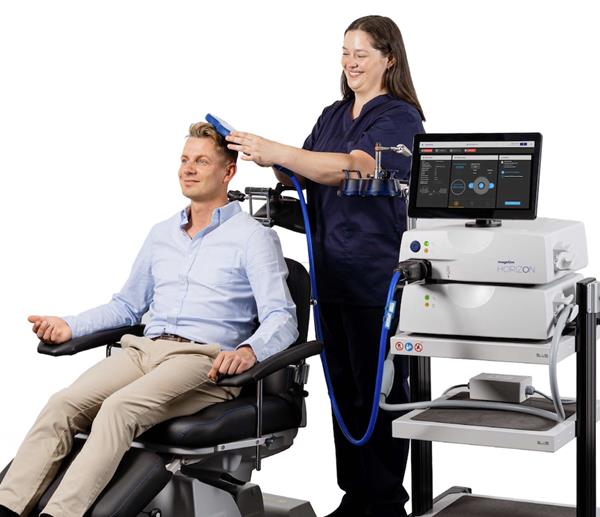
“The EU MDR is the most rigorous certification backed by verified scientific knowledge,” said Ronnie Stolec-Campo, CEO, Welcony. “As pioneers of neuromodulation for researchers and clinicians, our team is honoured to build world-class technology to study and treat the brain. This certification allows expanded reach to support this life-saving work.”
The European Union Medical Device Regulation (EU MDR) governs the safety, performance, and quality of medical devices sold in the European Economic Area (EEA). It replaced the previous Medical Device Directive (MDD). The certification is issued by a Notified Body, an independent organization designated by an EU member state.
“Our customers can be confident that with this certification, Magstim technology has passed some of the most rigorous quality testing medical devices are subjected to,” said Stolec-Campo. “The big thank you goes to our internal Welcony teams in Regulatory, R&D and support for your commitment to get this across the line—we know this will help people worldwide.”
As the only integrated TMS technology, Magstim is now the only TMS system which can treat MDD, Anxious Depression, OCD, and Adolescent Depression with a single treatment coil. The FDA recently cleared the Magstim Horizon 3.0 and Inspire Transcranial Magnetic Stimulation Systems for treating adolescent patients aged 15-21 for MDD, providing a non-invasive, non-pharmacological technology to improve care.
To learn more about the entire family of research and clinical neurotechnology innovations, visit Magstim.com or call 844-624-7846.
About Welcony
Globally, Welcony technologies have supported thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Interoperative Nerve Monitoring. Welcony is backed by Telegraph Hill Partners, a San Francisco based private equity company.
Media Contact: Mark Sejvar, msejvar@welcony.com, 323-363-3530
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6739d937-ac3a-4dc8-b8a8-f6e50bf48800
-
雷平阳走进“彩云英才荟”讲述 “云南故事“助力云南文旅产业发展(通讯员:徐龙云、李萍、 杨成林)近日,最新一期“彩云英才荟”——高层次人才服务与交流活动在昆明市中心翠湖之畔的百里·春秋春城会客厅(23°人才服务站)举行。著名诗2025-05-20
-
一文读懂低代码无代码平台:五大主流产品剖析如今,数字化转型已经不再是企业的可选项,而成为了必选项。低代码无代码平台也由此崭露头角,成为企业快速开发应用、提升效率的有力工具。这些平台通过可视化界面和简2025-05-20
-
何氏眼科完成首例新微创全飞秒SMILE pro手术!近视手术创新技术合作发布会成功举办5月18日,“尽享科技 感受极速”德国蔡司新一代机器人全飞秒VISUMAX 800 & 新微创全飞秒SMILE pro创新技术合作发布会在沈阳成功举行。本次发布会由沈阳何氏2025-05-20
-
熊大爷三款新面上市,开启舌尖美味新体验知名连锁餐饮品牌“熊大爷”宣布,近日正式推出三款全新面食——红烧牛肉面、双椒牛肉面及笋丁菇酱面。此次上新以“经典与创新融合”为亮点,通过差异化口味设计与高2025-05-20
-
宋章萌:光影逐梦人,内外皆璀璨在星光熠熠的演艺圈,宋章萌宛如一颗独特而耀眼的星辰,以扎实的演技与温暖的人格魅力,在银幕内外绽放光彩。 宋章萌出身于山东,齐鲁大地的文化2025-05-20
