Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces it has completed the acquisition of Integrity Orthopaedics, further strengthening its sports medicine shoulder repair business with the most comprehensive suite of technologies available for rotator cuff repair (RCR).
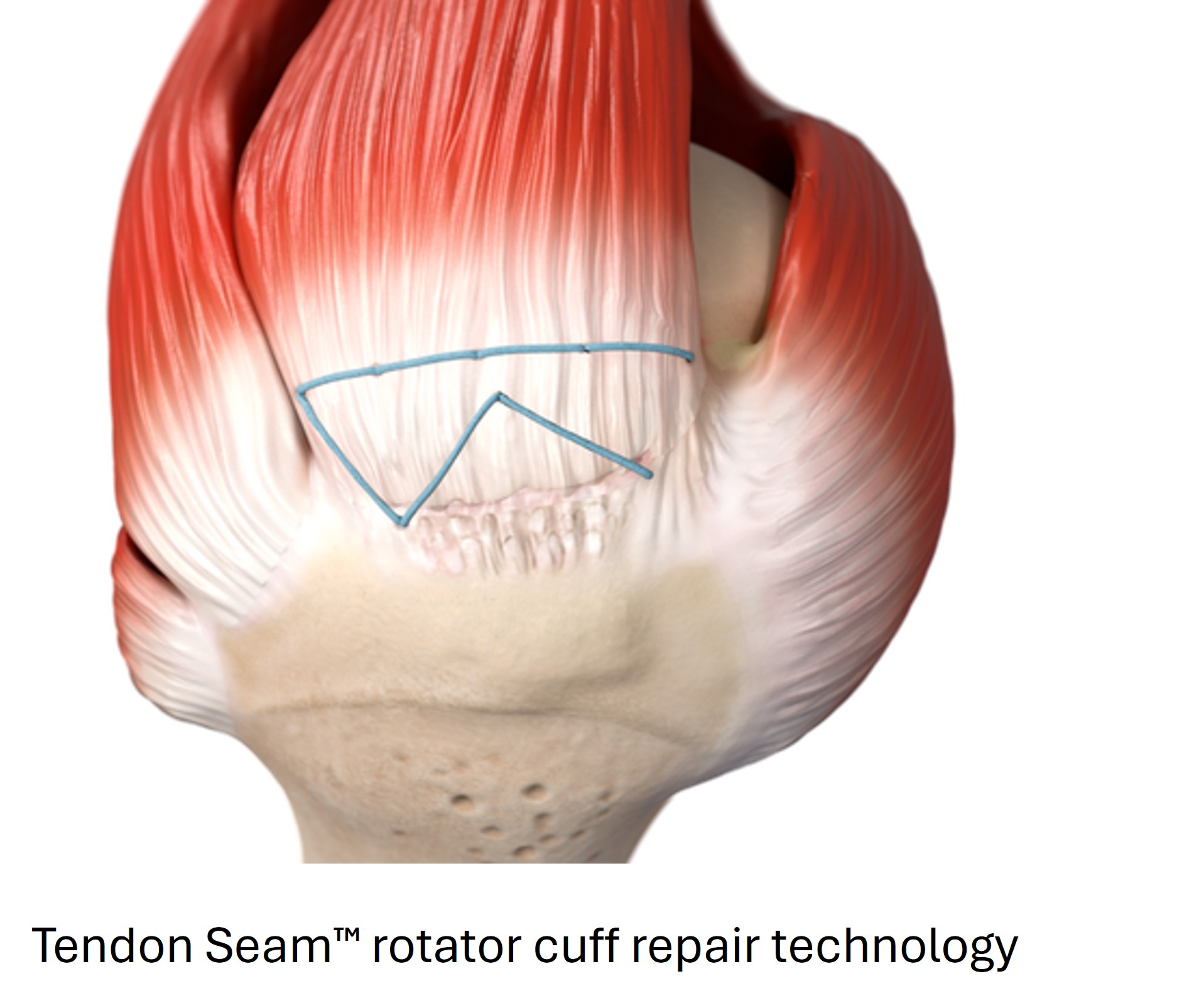
Integrity Orthopaedics is a US‑based, early‑stage commercial developer of Tendon Seam™, an innovative and disruptive biomechanical RCR system designed to significantly reduce re‑tear rates and improve patient outcomes versus the current standard of care.
Tendon Seam™ allows for a novel repair stitch configuration with multiple points of fixation. Its patented microanchors, continuous suture, individually locked stitches, and integrated implantation instrument are designed to deliver stronger repairs, accelerated patient recovery, lower re-tear rates, and a simplified surgical technique. Early clinical experience indicates low re-tear rates, reduced sling times, and shorter procedures compared with standard methods.1 The system received 510(k) clearance in 2023 and is indicated for reattachment of soft tissue to bone, including tendons, ligaments, and joint capsules.
Dr. Howard Harris, Orthopedic Surgeon at Texas Orthopedic Specialists and part of the Tendon Seam™ design surgeon team, said, “Arthroscopic biomechanical rotator cuff repair techniques using sutures and anchors have remained largely unchanged for over 15 years. Tendon Seam™ technology represents a significant innovation in rotator cuff repair technique. It gives me a more anatomic, stronger and efficient repair option that offers my patients a better chance of a successful recovery.”
RCR is a growing market segment with approximately 500,000 procedures performed annually in the US.2 Despite this, traditional surgical techniques have historically suffered from high structural failure rates, averaging 20–40%.3
Tendon Seam™ perfectly complements the market-leading REGENETEN Bioinductive Implant, giving Smith+Nephew a unique, differentiated and disruptive RCR portfolio. The two solutions represent the most advanced technologies currently available for biomechanical repair and biological augmentation – something no other company can offer.
“Surgeons and patients deserve a rotator cuff repair solution that delivers results they can count on,” said Christie Van Geffen, Senior Vice President of Sports Medicine Marketing for Smith+Nephew. “They now have that with Tendon Seam™ and REGENETEN - and we look forward to helping more tendon repairs heal, improving patient outcomes, and setting a new standard of care.”
Tendon Seam™ is currently available in the United States. Similar to post-acquisition efforts with the REGENETEN Bioinductive Implant, Smith+Nephew plans to accelerate adoption leveraging its commercial channels in the US, while building a base of evidence to support global adoption. To learn more about Tendon Seam™, please visit www.integrity-ortho.com.
- ends –
Media Enquiries
Dave Snyder +1 (978) 749-1440
Smith+Nephew david.snyder@smith-nephew.com
References
- Data on File at Integrity Orthopaedics.
- SmartTRAK, 2025, Shoulder Soft Tissue Fixation, 2025 BioMedGPS — SmartTRAK.
- Hein J, et al. Arthroscopy. 2015;31(11):2274-2281.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 17,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global business units of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in around 100 countries, and generated annual sales of $5.8 billion in 2024. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on X, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading profit margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: conflicts in Europe and the Middle East, economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and disposals, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; relationships with healthcare professionals; reliance on information technology and cybersecurity; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, which is available on the SEC’s website at www. sec.gov, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered in US Patent and Trademark Office.
-
成功平替国际巨头、韧性增长,嘉为蓝鲸筑牢IT研运行业的长期合作安全屏障成功平替国际巨头、韧性增长,嘉为蓝鲸筑牢IT研运行业的长期合作安全屏障2026-01-22
-
2026 功能食品健康峰会圆满落幕2026 年 1 月 21 日,为期两天的 2026 功能食品健康峰会(FFWS 2026)在泰国曼谷圆满落幕。作为亚太乃至全球功能食品领域的年度旗舰盛会与行业标杆性枢纽平台,本次峰会2026-01-22
-
世索科颁发30万欧元欧内斯特•索尔维奖予庄小威教授 表彰其开创性方法,揭示细胞在何处以及如何发挥功能并响应变化布鲁塞尔2026年1月22日 美通社 -- 世索科通过世索科基金会,将2026年欧内斯特•索尔维奖授予哈佛大学及霍华德•休斯医学研究所的庄小威教授,以表彰其在开发强大的成2026-01-22
-
迈威生物靶向 CDH17 ADC 创新药 7MW4911 完成美国临床试验首例患者给药上海2026年1月22日 美通社 -- 迈威生物(688062.SH),一家全产业链布局的创新型生物制药公司,宣布其靶向 CDH17 ADC 创新药(研发代号:7MW4911)用于晚期结直肠癌及其他晚期2026-01-22
-
重磅喜讯丨 山王果隐龙刺梨酵活原液斩获“健观奖·2025年度健康产品”重磅喜讯丨 山王果隐龙刺梨酵活原液斩获“健观奖·2025年度健康产品”2026-01-22
